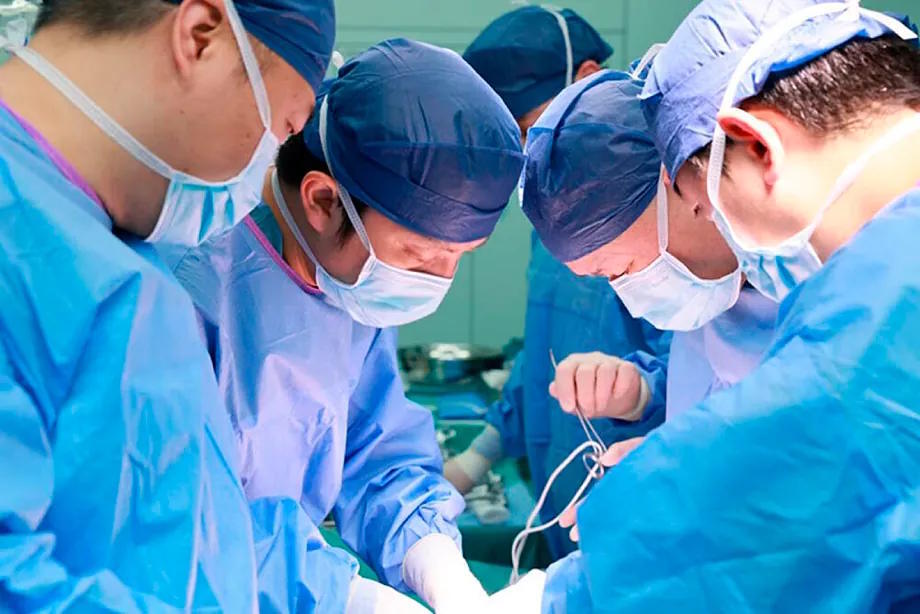
In the operating room, the patient lay connected to a machine while, next to them, on a side table, a genetically edited pig liver filtered their blood through a circuit of tubes. Thus, for hours, an animal organ assumed functions that the patient's human liver could no longer perform. It was not a traditional transplant: the organ was not implanted but working from the outside, like a biological bridge to buy time.
Doctors at Xijing Hospital in the Chinese city of Xi'an silently watched the monitors. Each hour gained increased the chances of the patient surviving long enough to receive a transplant or for their own liver to start recovering. Suddenly, toxin levels began to decrease. The parameters gradually improved. The patient was coming out of liver failure.
Chinese state television has been broadcasting some images this week of a successful clinical trial announced in early February, marking a new step in the race to use animal organs in humans, a line of research that China has turned into one of its most ambitious medical endeavors due to the chronic shortage of donors.
"It is the first time that an extracorporeally maintained pig liver has managed to reverse acute liver failure in a human patient," states a note from the Chinese biotechnology company ClonOrgan, which was responsible for editing six genes of the pig liver used in the procedure to increase its compatibility with human tissue and improve blood clotting.
The pig liver, explain the researchers, was placed in a perfusion device and connected to the patient's circulatory system through an external circuit. For 66 hours, the organ performed essential functions: detoxification, protein synthesis, and metabolism, basic tasks normally carried out by the human liver.
According to the hospital, the pig liver functioned well, producing approximately one-third of the bile that the human organ produces. Nearly three days after the procedure, key indicators of the patient's liver function had improved. The latest information, as of February 5, indicated that the patient's vital signs were stable, with physiological and biochemical indicators approaching normal levels.
"The system, in practice, functions as a bridge: it stabilizes the patient while waiting for a human transplant or while their own liver recovers. This approach reduces immune rejection, one of the major obstacles of xenotransplantation, because the organ does not remain inside the body," details surgeon He Xiaoshun, one of the most cited specialists in this field and director of a major transplant program in Guangzhou.
He emphasizes that the main goal of these trials is not yet to completely replace human organs but to understand how genetically modified organs interact with the human body in real clinical conditions.
In March 2024, surgeons at Xijing Hospital successfully implanted the first pig liver into a patient in a vegetative state to study its functioning in real conditions. The organ, with six genes edited to reduce immune rejection and improve compatibility with human blood, remained active for ten days.
During that time, doctors confirmed that the liver produced bile, synthesized essential proteins, and maintained basic metabolic functions without signs of hyperacute rejection, the main historical obstacle of xenotransplants. The experiment was designed as a viability test: it did not aim to save the patient but to observe to what extent an animal organ could assume complex physiological tasks in the body for extended periods.
That same year, a team from the University of Guangzhou performed the first experimental lung transplant from a pig into a 39-year-old man in a brain-dead state. For several days, the organ partially functioned and allowed monitoring of its viability, but the patient developed generalized swelling and fluid accumulation in tissues, likely due to blood flow issues. Although there were signs of partial recovery, doctors soon observed that the body was starting to reject the organ.
The most ambitious leap came shortly after. In 2025, the Journal of Hepatology published the case of a 71-year-old patient with advanced cirrhosis and liver cancer who survived nearly six months after receiving a genetically modified pig liver at the Department of Hepatobiliary Surgery at Anhui Medical University.
This was the first hepatic xenotransplant performed in a living human recipient with prolonged follow-up. Doctors managed to maintain the graft function for 171 days, a time that, although not a definitive cure, demonstrated that an animal organ could sustain human life for months.
"The combined use of genetically edited organs and extracorporeal circulation systems can provide temporary life support and greatly reduce the need for aggressive immunosuppression," explains now Tao Kaishan, a surgeon at Xijing Hospital who participated in the recent trial. Pigs have become the preferred animal for this type of research because their organs have a size and physiology similar to humans, reproduce rapidly, and can be genetically modified relatively easily.
Most specialists insist that, for now, the immediate goal is not to completely replace human transplants but to buy time: crucial days or weeks for a donor to appear or for the patient's own organ to recover.
But Wang Lin, director of hepatobiliary surgery at Xijing Hospital, argues that these techniques "can not only give more time to patients on waiting lists but also expand treatment options for end-stage liver failure." According to Wang, the results indicate that pig livers could potentially "replace or support human liver function" in certain clinical scenarios, although there is still a long way to go.