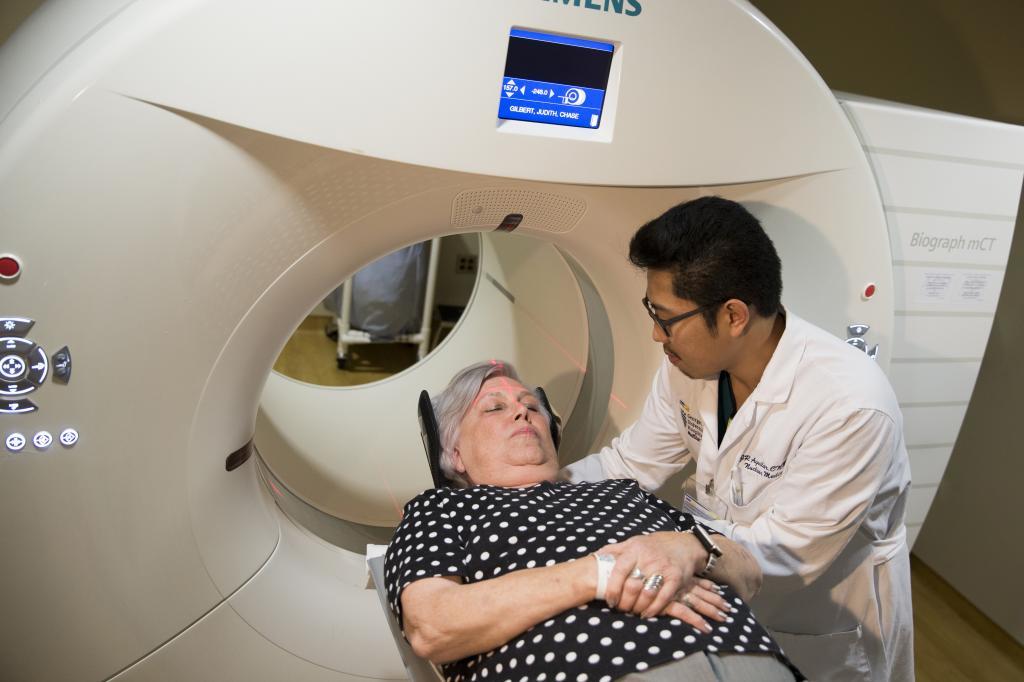
Fluorescence microscopy images of a mouse brain 12 hours after receiving the nanoparticles under study. In red, beta-amyloid plaques, and in green, the blood vessels of the blood-brain barrier.IBEC
An innovative experiment explores the possibility of treating Alzheimer's by modulating the blood-brain barrier, rather than with current strategies. The research, co-led by scientists from the Institute of Bioengineering of Catalonia (IBEC) and the West China Hospital of Sichuan University, has achieved in the murine model of the disease, the clearance of beta-amyloid plaques and cognitive recovery through nanoparticles that target the brain vasculature.
For this, only three injectionsof the nanoparticles were needed, which acted as bioactive substances, not just as vectors of a therapeutic molecule. These "supramolecular drugs" restored the proper functioning of the blood-brain barrier, resulting in a recovery of disease symptoms in the animals.
"We created a nanoparticle capable of acting on the brain vasculature, through which the brain receives nutrients and eliminates toxic substances from its interior," explains the lead author of the research Giuseppe Battaglia, ICREA Research Professor at IBEC, where he is the principal investigator of the Molecular Bionics Group.
"This is one of the problems that arise in many dementias, such as Alzheimer's disease, where proteins and toxic substances accumulate and cause serious damage," the scientist points out in a video released by IBEC. "Our nanoparticle can reactivate the natural elimination of these substances. The results were highly promising: we demonstrated that after a single injection, the level of toxic substances [beta-amyloid protein] in the brain was reduced by 50%. After three injections in a relevant animal model of this pathology, we demonstrated a complete reversal of the disease progression, with an effect that lasted the equivalent of 20 human years. It is very exciting and will provide us with a lot of work for its future development," Battaglia confides.
The new generation of drugs and diagnostics opens a new era in Alzheimer's: "We must not forget the unchanging needs"
The innovative proposal of an Alzheimer's 'spin-off' from the Cajal Institute: gene therapy to fight neurodegeneration
Also highlighting the observed recovery is Lorena Ruiz Pérez, researcher in the Molecular Bionics group at IBEC and adjunct professor Serra Hunter at the University of Barcelona, who states that "the results are very promising, as one hour after the administration of the first injection, we see a reduction in this accumulation of beta-amyloid of 50 to 60% in animal models. After the complete dose, which is three injections, we have seen a recovery of cognitive abilities in animal models that lasts over time and is the equivalent of about 20 to 30 years in people, meaning that we restore normal brain function equivalent to a healthy person without the disease." A mouse, six months after the injections, at 18 months of age (comparable to a 90-year-old human), had regained the behavior of a healthy mouse.
Elimination into the bloodstream
The strategy tested in this international multicenter research, details of which are published in Signal Transduction and Targeted Therapy, focuses on the blood-brain barrier, the cellular and physiological wall that separates the brain from the bloodstream to protect it from the harmful action of pathogens or toxins. Proper functioning depends on the adequate elimination of toxic proteins into the bloodstream; something that does not occur in Alzheimer's disease, where beta-amyloid protein is deposited in plaques damaging neurons.
"The long-term effect comes from the restoration of the brain's vascular system. We believe it works like a cascade: when toxic species like beta-amyloid accumulate, the disease progresses. But once vascularization can function again, it starts to eliminate beta-amyloid and other harmful molecules, allowing the entire system to regain its balance. The most relevant thing is that our nanoparticles act as a drug and seem to activate a feedback mechanism that returns this elimination pathway to normal levels," explains Battaglia.
For the brain to eliminate the toxin, the LRP1 protein acts as a molecular guardian: it recognizes beta-amyloid protein, binds to it with ligands, and transports it through the blood-brain barrier to the bloodstream, where it is eliminated. However, this function depends on a delicate balance of protein binding that can be altered by excess or defect.
The nanoparticles developed by these researchers act as a switch that restores the system, to return the balance that allows the proper elimination of beta-amyloid protein and thus prevent its accumulation in plaques. Designed with a bottom-up molecular engineering approach, the nanoparticles combine precise size control with a defined number of ligands on the surface, creating a multivalent platform capable of interacting with cell receptors in a very specific way. By participating in receptor trafficking on the cell membrane, they open a unique and novel way to modulate receptor function. This precision not only allows for the effective removal of beta-amyloid protein from the brain but also restores the balance of the vascular system to maintain its normal brain function.